Abstract
Introduction. Immunoparesis (IP) is defined as the suppression of uninvolved immunoglobulins (Ig) and is a very frequent finding in multiple myeloma (MM) patients at diagnosis. The classical and most common way to measure it is by nephelometry of other immunoglobulins (classic IP), but also in recent years we can measure the uninvolved heavy/light chain pair of the same immunoglobulin (uHLC). Recovery of classic IP have been reported as a good prognosis factor in some studies, but these are observational retrospective works with non-homogeneous treatment and most of them with a non-continuous treatment. Recovery of uHLC IP have not been correctly studied as far as we know. Minimal residual disease (MRD) has become in the last years probably the most important prognosis factor during evolution.
Aim. Evaluate the prognostic value of IP recovery by classic Ig and uHLC, in terms of progression free survival (PFS), within a clinical trial of newly diagnosed MM transplant-eligible (NDMM TE) patients with intensive continuous treatment and evaluate the association with MRD.
Patients & Methods. Patients with newly diagnosed MM enrolled in the PETHEMA/GEM2012MENOS65 trial received six cycle VRD-GEM induction, autologous hematopoietic stem cell transplantation conditioned by melphalan or busulfan plus melphalan and consolidation with two more cycles of VRD-GEM. Afterward, patients were enrolled in the PETHEMA/GEM2014MAIN clinical trial that randomly assigned them to maintenance with lenalidomide and low-dose dexamethasone (Rd) or Rd plus ixazomib. We analyze classic Ig and uHLC in a centralized laboratory at diagnosis, after consolidation treatment and at the first year of maintenance. We consider IP at diagnosis when one or more uninvolved classic Ig or uHLC were under lower limit of normality (LLN) and Recover IP when one or all classic Ig who were suppressed at diagnosis or suppressed uHLC at diagnosis reach at least LLN plus 10%. MRD was analyzed by next generation flow cytometry after consolidation (sensitivity level ≥10-5). High risk cytogenetics was defined as del17p, t(4;14), t(14;16) and/or +1q21. We included in this study 245 patients with samples available at any of the three time points for the analysis.
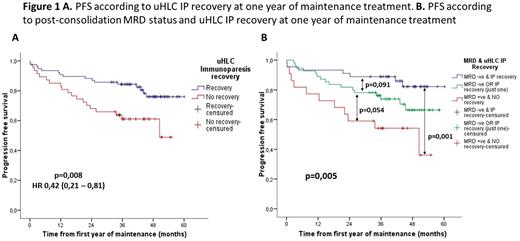
Results. At diagnosis we found classic IP in 86,7% of patients (203/234) and uHLC IP in 94,5% of patients (191/202). No difference in PFS was found between patients with or without IP at diagnosis for both methods. After consolidation we found recovery of classic IP in 42,4% of patients (87/205) and recovery of uHLC in 49,2% of patients (89/181). We also found no differences in PFS between patients who recover or not recover IP for both methods. After the first year of maintenance, we found recovery of classic IP in 53,8% (77/143) and recovery of uHLC in 63,2% of patients (84/133). We found no differences for recovery of classic IP, but patients with recovery of uHLC IP after one year of maintenance had better PFS (p=0,008) with hazard ratio 0,42 (Figure 1A). When we include recover uHLC IP with MRD after consolidation and high-risk cytogenetics at diagnosis in a multivariate analysis for PFS we confirm that all three variables maintain their independent prognosis value for PFS.
We stratified patients in three categories according to post-consolidation MRD status and uHLC recovery after one year of maintenance. As we can see in Figure 1B patients with both favorable factors (MRD negative and recover IP) had better PFS (p=0,001) than patients with both unfavorable factors (MRD positive and no recover IP). Patients with only one favorable factor (MRD negative or recover IP) seems to have an intermediate risk status but differences within the other groups were not statistically significative (p=0,091 and p=0,054 respectively).
Conclusions. Recovery of uHLC IP after one year of maintenance is an independent prognostic factor for PFS in NDMM TE patients and intensive continuous treatment. Classic Ig are less sensitive than uHLC for measure immune reconstitution in this scenario. Immune reconstitution, measured as recovery of uHLC IP, may provide complementary prognostic value to MRD.
Disclosures
Lakhwani:Janssen: Honoraria, Other: Advisory board; Novartis: Honoraria. Rosinol Dachs:BMS-Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; GlaxoSmithKline: Honoraria. Puig:Celgene, Janssen, Amgen andTakeda: Research Funding; Amgen, Celgene, Takeda and The Binding Site: Honoraria; Amgen, Celgene, Janssen and Takeda: Consultancy; Celgene: Honoraria, Speakers Bureau. Paiva:Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; EngMab: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Roche Glycart AG: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Adaptive: Honoraria; Amgen: Honoraria; Gliead: Honoraria; Oncopeptides: Honoraria. Oriol:BMS: Consultancy, Speakers Bureau; Janssen: Consultancy; GlaxoSmithKline: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau. Rios:Becton-Dickinson, Celgene, GSK, Janssen, Sanofi, Binding Site: Consultancy. Jarque:Amgen: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; BeiGene: Consultancy; Gilead: Consultancy, Speakers Bureau; Grifols: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Speakers Bureau; Kiowa Kirin: Consultancy; Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Shionogi: Consultancy; Sobi: Consultancy; Takeda: Consultancy, Speakers Bureau. Moraleda:Novartis, Gilead, Roche, Sanofi, Jazz, and Takeda.: Consultancy. Sureda:ROCHE: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; BMS: Consultancy, Honoraria; MSD: Honoraria; TAKEDA: Consultancy, Honoraria, Speakers Bureau; SANOFI: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; GILEAD: Consultancy. Garcia:Janssen, Celgene (BM), Takeda: Consultancy; Janssen ,Celegene (BM), Takeda, GSK , Sanofi, Amgen: Honoraria, Speakers Bureau. Casado Montero:Janssen: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Loxo: Research Funding. Encinas:Bristol Myers Squibb: Honoraria; GlaxoSmithKline: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Pfizer: Honoraria; Sanofi: Honoraria, Speakers Bureau. De Arriba:Amgen: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; Glaxo Smith Kline: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau. Sampol:GSK: Consultancy. Alonso Fernández:Janssen: Honoraria; BMS: Honoraria; Pfizer: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Amgen: Honoraria. Mateos:Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides, Seagen: Honoraria. Bladé Creixenti:Janssen, Calegen/BMS,Amgen, Takeda, Oncopeptides: Honoraria. San-Miguel:Abbvie, Amgen, BMS, Celgene, GSK, Haemalogix, Janssen-Cilag, Karyopharm, MSD, Novartis, Takeda, Regeneron, Roche, Sanofi, and SecuraBio: Consultancy, Other: Advisory Board. Hernández:Roche, GSK: Other: Advisory board; Sanofi, -amgen, Janssen, Celgene (BMS): Honoraria, Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal